Multidisciplinary Products at Biomedical Engineering ITB
By Adi Permana
Editor Adi Permana
BANDUNG, itb.ac.id—Prof. Dr. Ir. Tati Latifah R. Mengko stated that Biomedical Engineering is a multidisciplinary field which utilizes various concepts of engineering and design in medicine and biology to enhance health services and people’s quality of life.
Products successfully produced by Biomedical Engineering ITB are classified into three categories, namely final products that are about to enter the market, products in the process of development and handled by a small unit, and products developed and handled by a large unit.
According to the Head of the Biomedical Engineering Research Group ITB, one of the products that have entered the market is the Tele-EKG device, a product originated from a collaboration with TESENA and Pusat Jantung Nasional (National Cardiovascular Center).
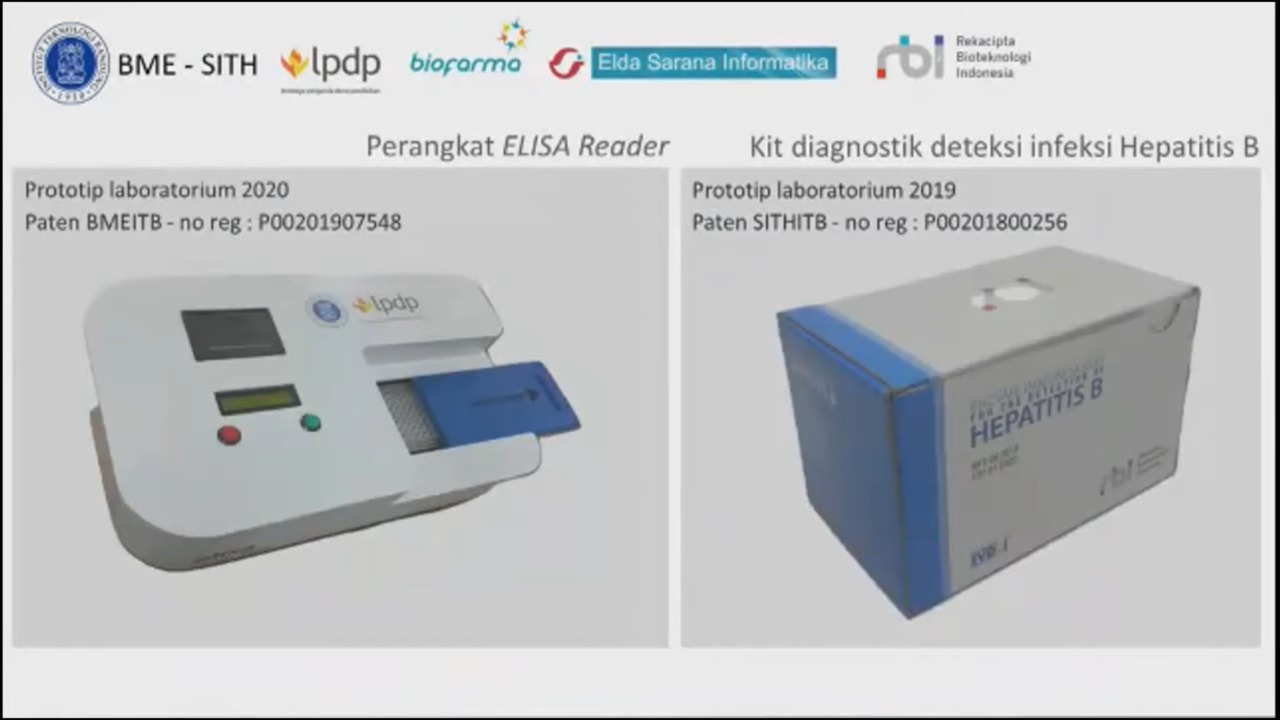
In addition, other than the Tele-EKG device, there are other products and developments being worked on such as ELISA reader, a diagnostic kit to detect Hepatitis B infection, the EKG-lysis device, Diaretino which is a diabetes mellitus detection device, and Vretino which is an optic nerve measurement instrument. Products currently being developed are among others a software application for medical check-up during COVID-19 self-isolation, medical waste management product, and TBC scanning microscope, which is the fruit of collaboration between ITB, UNAIR, and MIT.
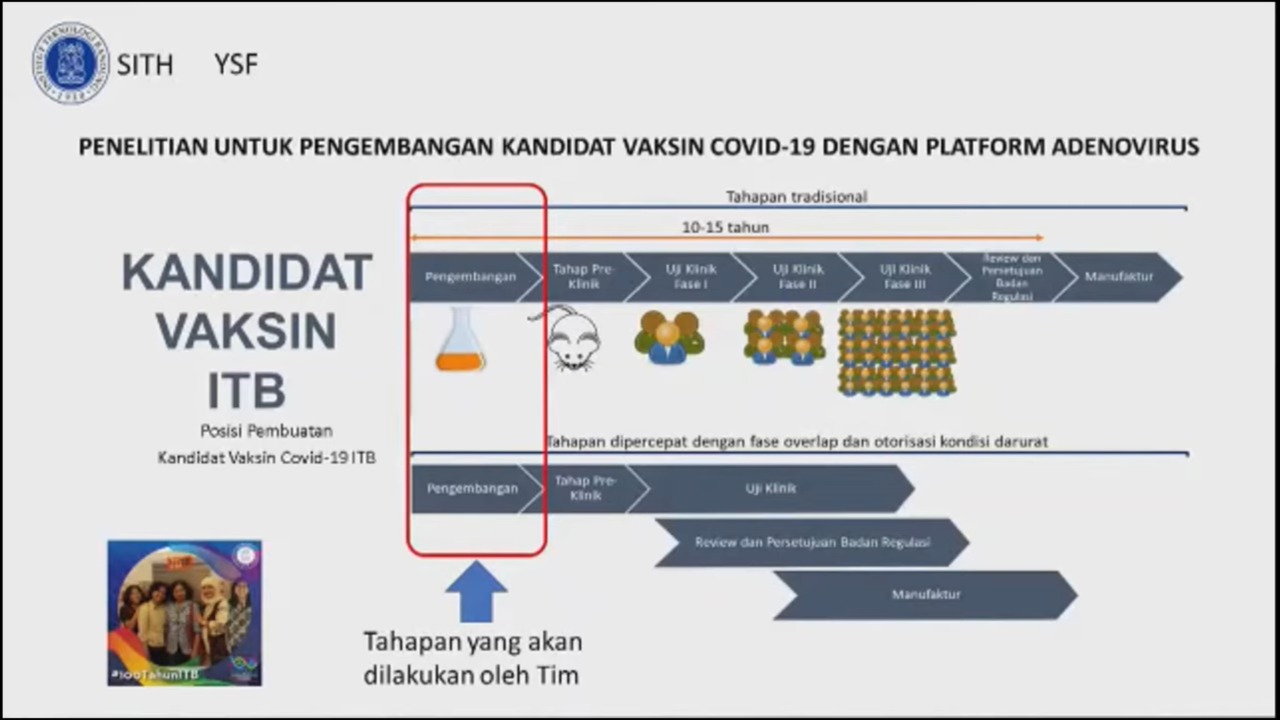
“Other than the products generated from collaborations between Biomedical Engineering ITB and other institutions, some Faculties at ITB have successfully developed products in the realm of biomedicine. Among them are Exo-skeleton and Bionic Hand from the Faculty of Mechanical and Aerospace Engineering (FTMD), Ventinesia from Engineering Physics (TF), research in the field of protein expression and research on the development of COVID-19 vaccine candidates with the adenovirus platform by School of Life Sciences and Technology (SITH), the HPV antigen protein vaccine, therapeutic protein, and diagnostic enzyme for qPCR by School of Pharmacy (SF), as well as the development of rapid test and recombinant antigens by the Faculty of Mathematics and Natural Sciences ITB (FMIPA),” she added.
During the innovation process, differences may emerge between partners who conduct research and those who will distribute the products to the market. The form of required support and competence between the two also differs. In most cases, many innovations are found to be falling to the ‘valley of death’. This is a point in which innovation products are just about to enter the market. Prof. Tati hoped that the current products being worked on can successfully pass this phase so that they can finally be introduced to the market.
“Essentially it is (all about) working together, having mutual trust, and mutual recognition that will encourage (everyone) to go forward,” explained Prof. Tati regarding the collaboration culture at ITB internally and with the external parties, which was delivered during the ITB Talks event. She also emphasized that the impact of collaborations in the product innovation stage will give a significant advantage, especially in the product development and testing stages, as it can accelerate the entry of these products to the market for it to be accepted by the population.
Reporter: Rizana Salsabila
Translator: Rizvika Rahmita Salim

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
