Biotechnology Industry: From Skin to Vaccine
By Adi Permana
Editor Adi Permana

BANDUNG, itb.ac.id – Biotechnology plays a big role in many aspects of human life, especially in industry, medicine, and agriculture. On Monday (15/3/2021), Master Program of SITH ITB held a seminar series under the title of “Biotechnology Fair” with two keynote speaker, Sharlini Eriza Putri and Dr. Yusuf Sofyan. Biotechnology in industry field is the main topic of the first day’s Serie of seminar.
The Microbiome in Skin Health
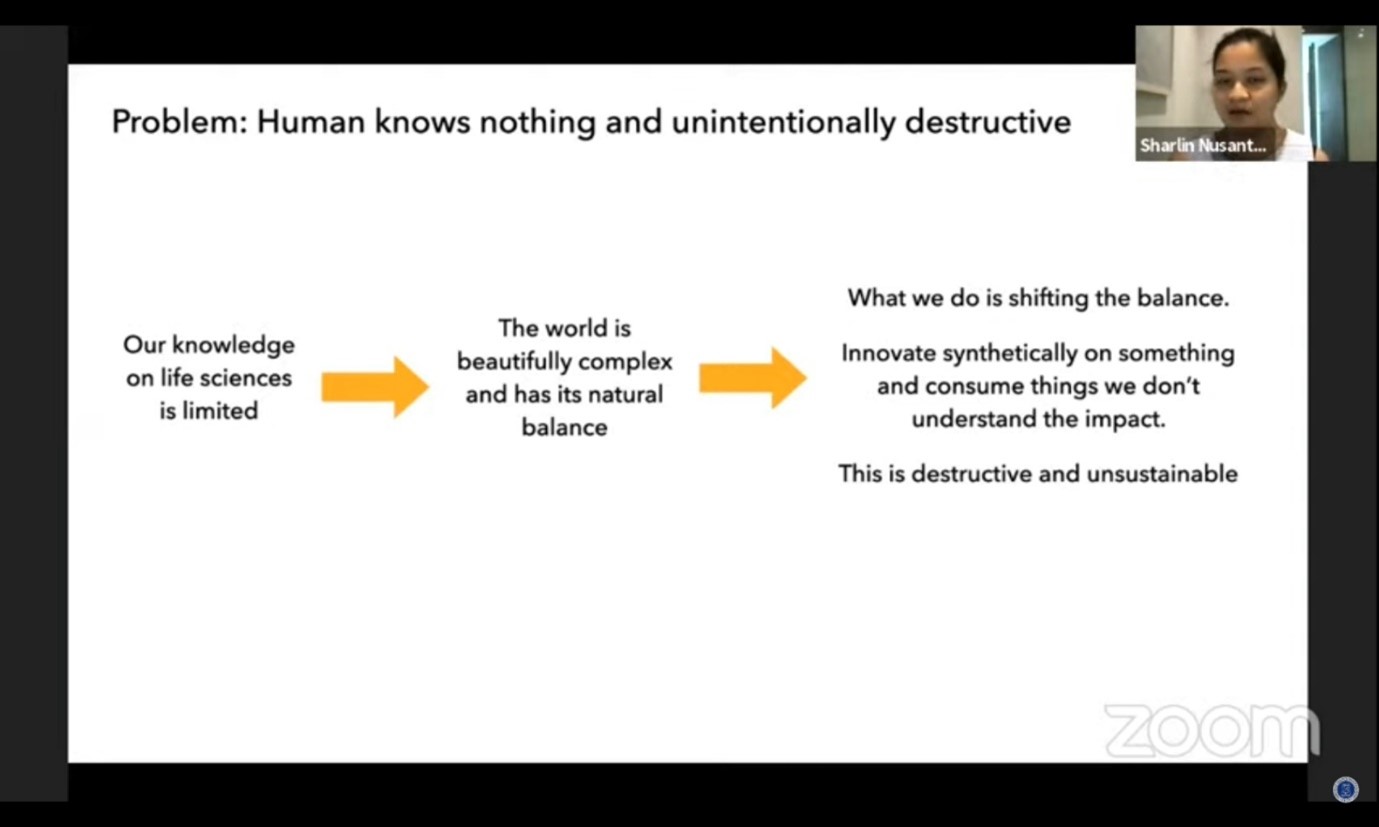
According to CEO of Nusantics, Sharlini Eriza Putri, society often perceive their skin as “sofa” which can be cleaned just by wiping it when the surface is dirty. Whilst in reality, skin is much more complex than that. Skin is likened as earth’s surface with numerous microbes live on it. Those microbes share a symbiotic relationship to each other and not all of them give a bad influence towards skin.
“The consistency, wherever it is, the more diverse the microbiome on your skin, the more skin problems became invisible”, she said.
Ironically, all substances that applied on skin will “shift” the microbiome equilibrium. Whereas, according to Sharlin, what skin need to survive naturally is microbiome balance. Therefore, to maintain the microbiome balance on skin, we better only use what our skin needs. “What we should do is use something simple, only the necessary,” she explained.
Vaccine Development: From Lab Bench to Manufacturing
As an opening to this session, Dr. Yusuf explained that there are three main categories based on parts that used to produce vaccine: whole bacteria, parts of virus or bacteria that triggers immune system, and genetic material.
Based on Research Coordinator on Bacteria and Recombinant Vaccines Biofarma, Dr. Yusuf Sofyan Efendi elucidation, lab bench or steps in producing vaccine begin with determining antigen, then formulating it and combined with other components. The formula or the combination then will finally be tested on animal-or referred as preclinical assays. If the result is coming out safe, the try-out vaccine will take a further test.
“Not all vaccine candidates can pass preclinical assay, only a few that could continue up until licensing stage”, he continued. This is due to the majority of technological platform which developed gave not enough immune response.

He explained, when a vaccine candidate looks promising, there will be a production process in lab scale to be tested on human, guided by strict rules from BPOM (National Food and Drug Agency). This production process itself went through a lot of stages, start from producing the materials, enclosing analysis certification, methods, and the tests that being done.
He then further explained that vaccine went through multiple sterilization step, continues with upstream production and downstream production afterwards. Quality control and clinical testing in three stages also became the crucial part in vaccine manufacturing process, before finished in registration and prequalification from WHO (World Health Organization).
Reporter: Athira Syifa (Teknologi Pascapanen, 2019)
Translator: Aghisna Syifa Rahmani (TPB-SITHS 2020)

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)


