Vent-I Portable Ventilator by Indonesian Young Generation Passes the Test of Indonesian Ministry of Health and Ready for Mass Production
By Vera Citra Utami
Editor Vera Citra Utami
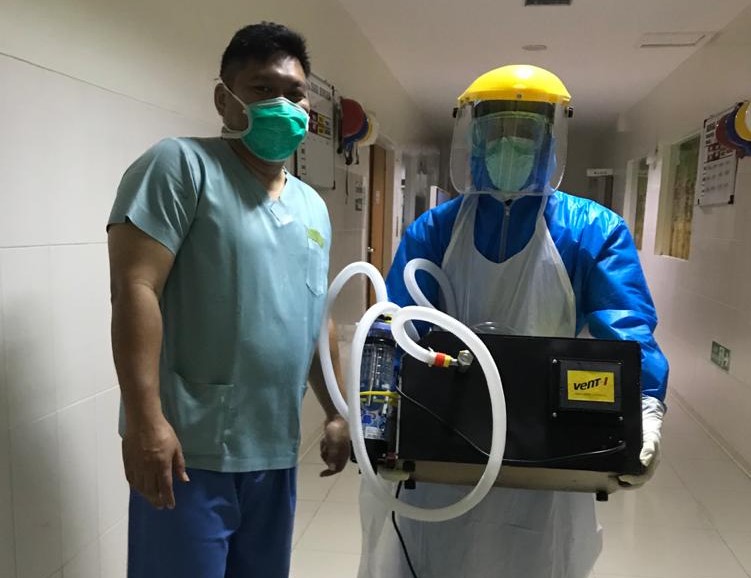
Following the completion of a comprehensive product testing process by the Health Facilities Safety Center (BPFK) of Indonesian Ministry of Health, CPAP portable ventilator products, Vent-I was declared to have passed the test successfully on 21 April 2020.
Products resulting from the collaboration between Institut Teknologi Bandung (ITB), Universitas Padjadjaran (UNPAD) and YPM Salman have been declared to have passed the tests for all test criteria in accordance with SNI IEC 60601-1: 204: General Requirements for Basic Safety and Essential and Rapid Performance Manufactured CPAP Systems, Document CPAP 001, Specification, MHRA, 2020.
The ventilator was initiated by Dr. Syarif Hidayat, lecturer at the School of Electrical and Information Engineering (STEI) of the ITB Electricity Expertise Group, supported by several lecturers and students from the Faculty of Mechanical and Aerospace Engineering (FTMD) and Product Design, Faculty of Fine Arts and Design (FSRD). Vent-I is a breathing apparatus for patients who are still able to breathe on their own (if the COVID-19 patient has stage 2 clinical symptoms) and not for patients with ICU. Vent-I is said to be easily used by medical personnel. The instrument has the main function of CPAP (Continuous Positive Airway Pressure).
By passing this product test, Vent-I was declared safe to be used as a non-invasive ventilator to assist COVID-19 patients. This has been conveyed directly to Ir. Hari Tjahjono, MBA., As the Public Communications Team of the Vent-I Developer, published by ITB Public Relations, Wednesday (4/22/2020).

Vent-I can be produced immediately for social purposes, where Vent-I will be distributed free of charge to hospitals in need. "As a result of this social need, Vent-I will be produced around 300-500 according to the amount of donations received by Salman Charity House. The first phase of production will begin when the test is passed on April 21 yesterday and will be produced in collaboration with PT DI," Hari said when contacted by the ITB Public Relations Reporter.
In addition, Vent-I will be used in patients with medical indications and will be accompanied by the Association of Indonesian Anaesthesiologists and Intensive Therapy Specialists (Perdatin) in designated hospitals. "As for commercial purposes involving the purchase and sale of transactions, the marketing authorisation is currently in the administrative process, which is expected to be ready in the next few days. This activity will be managed by PT. Rekacipta Inovasi ITB," Hari said.
The Vent-I Development Team also expressed its deep appreciation and appreciation to all parties, so that the first ventilator produced by this nation's children could pass the test in accordance with the medical device standards set by the Government. "Special thanks go to the donors who have donated funds for the development of Vent-I since the design stage so far. Without financial support from the donors, this project would not have been possible," he said.
In addition , the team also thanked the BPFK team, Indonesian Ministry of Health, for their hard and proactive work in providing technical guidance and conducting the test process professionally so that the test process could be carried out in a very short time. "Hopefully, our efforts to develop the first ventilator of this nation's generation can help to better manage COVID-19 patients," Hari concluded.
Reporter: Adi Permana
Translator: M. Akbar Selamat
Translator: M. Akbar Selamat

.jpg)

.jpg)
.jpg)
.jpg)